Nori Salmon TLR5 ELISA Kit
$508.00 – $916.00
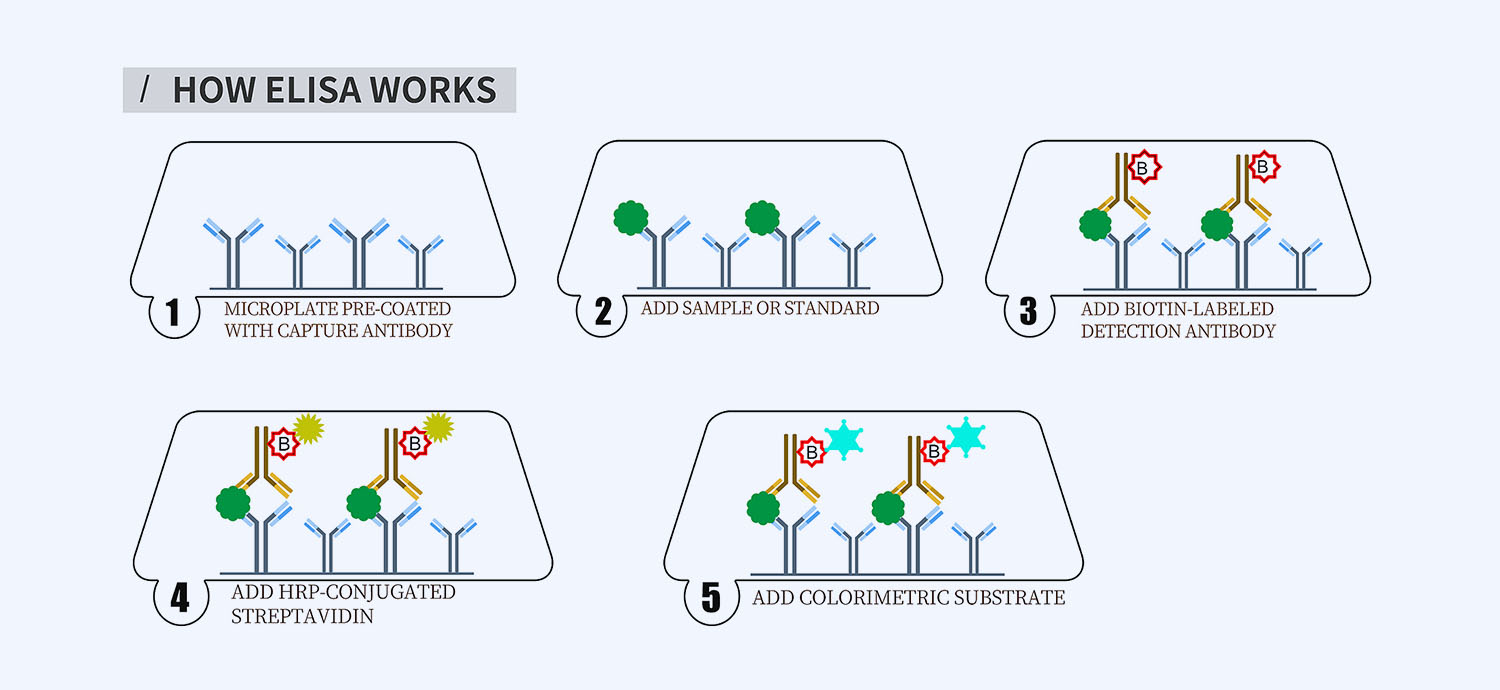
This ELISA kit is for quantification of TLR5 in salmon. This is a quick ELISA assay that reduces time to 50% compared to the conventional method, and the entire assay only takes 3 hours. This assay employs the quantitative sandwich enzyme immunoassay technique and uses biotin-streptavidin chemistry to improve the performance of the assays. An antibody specific for TLR5 has been pre-coated onto a microplate. Standards and samples are pipetted into the wells and any TLR5 present is bound by the immobilized antibody. After washing away any unbound substances, a detection antibody specific for TLR5 is added to the wells. Following wash to remove any unbound antibody reagent, a detection reagent is added. After intensive wash a substrate solution is added to the wells and color develops in proportion to the amount of TLR5 bound in the initial step. The color development is stopped, and the intensity of the color is measured.
Alternative names for TLR5: toll-like receptor 5
This product is for laboratory research use only not for diagnostic and therapeutic purposes or any other purposes.
- Description
- How Elisa Works
- Product Citation
- Reviews (0)
Description
Nori Salmon TLR5 ELISA Kit Summary
Alternative names for TLR5: toll-like receptor 5
Alternative name for salmon: salmo salar, atlantic salmon
| Assay Type | Solid Phase Sandwich ELISA |
| Format | 96-well Microplate or 96-Well Strip Microplate |
| Method of Detection | Colorimetric |
| Number of Targets Detected | 1 |
| Target Antigen Accession Numbe | A0A1S3MAI0 |
| Assay Length | 3 hours |
| Quantitative/Semiquantitative | Quantitative |
| Sample Type | Plasma, Serum, Cell Culture, Urine, Cell/Tissue Lysates, Synovial Fluid, BAL, |
| Recommended Sample Dilution (Plasma/Serum) | No dilution for sample <ULOQ; sufficient dilution for samples >ULOQ |
| Sensitivity | 50 pg/mL |
| Detection Range | 0.25-16 ng/mL |
| Specificity | Salmon TLR5 |
| Cross-Reactivity | < 0.5% cross-reactivity observed with available related molecules, < 50% cross-species reactivity observed with species tested. |
| Interference | No significant interference observed with available related molecules |
| Storage/Stability | 4 ºC for up to 6 months |
| Usage | For Laboratory Research Use Only. Not for diagnostic or therapeutic use. |
| Additional Notes | The kit allows for use in multiple experiments. |
Standard Curve
Kit Components
1. Pre-coated 96-well Microplate
2. Biotinylated Detection Antibody
3. Streptavidin-HRP Conjugate
4. Lyophilized Standards
5. TMB One-Step Substrate
6. Stop Solution
7. 20 x PBS
8. Assay Buffer
Other Materials Required but not Provided:
1. Microplate Reader capable of measuring absorption at 450 nm
2. Log-log graph paper or computer and software for ELISA data analysis
3. Precision pipettes (1-1000 µl)
4. Multi-channel pipettes (300 µl)
5. Distilled or deionized water
Protocol Outline
1. Prepare all reagents, samples and standards as instructed in the datasheet.
2. Add 100 µl of Standard or samples to each well and incubate 1 h at RT.
3. Add 100 µl of Working Detection Antibody to each well and incubate 1 h at RT.
4. Add 100 µl of Working Streptavidin-HRP to each well and incubate 20 min at RT.
5. Add 100 µl of Substrate to each well and incubate 5-30 min at RT.
6. Add 50 µl of Stop Solution to each well and read at 450 nm immediately.
Background:
Toll-like receptor 5 (TLR5) is a protein which is encoded by the TLR5 gene.[1] TLR5 is known to recognize bacterial flagellin from invading mobile bacteria. It has been shown to be involved in the onset of many diseases, which includes Inflammatory bowel disease.[2] Recent studies have also shown that malfunctioning of TLR5 is likely related to rheumatoid arthritis,[3] osteoclastogenesis, and bone loss.[4] Abnormal TLR5 functioning is related to the onset of gastric, cervical, endometrial and ovarian cancers.[5] TLR5 is expressed on both immune and non-immune cells. The activation of this receptor mobilizes the nuclear factor NF-κB and stimulates TNF alpha production. There are highly conserved regions in the flagellin protein among all bacteria, facilitating the recognition of flagellin by a germ-line encoded receptor such as TLR5.[6] However, some Proteobacteria flagella have acquired mutations preventing their recognition by TLR5.[7] The TLR5 signaling cascade is commonly triggered by the binding of bacterial flagellum to TLR5 on the cell surface. Binding of flagellum induces the dimerization of TLR5, which in turn recruits MyD88.[8] The recruitment of MyD88 leads to subsequent activation of IRAK4, IRAK1, TRAF6, and eventually IκB kinases.[9] Activation of IκB kinases contributes to the nuclear localization of NF-κB. NF-κB induces many downstream gene expressions, which initiates the canonical proinflammatory pathway. This TLR5/flagellum interaction results in different responses in different cell types. In epithelial cells, binding of flagellum to TLR5 induces IL8 production. In human monocytes and dendritic cells, this interaction results in the secretion of proinflammatory cytokines such as TNF. Recent study has identified Caveolin-1 as a potential regulator of TLR5 expression.[10] In contrast to the decreased TLR4 level in senescent cells, TLR5 expression maintains relatively stable during the aging process, which is correlated with the high level of Caveolin-1 in aging cells. Data from Caveolin-1 knockout mice demonstrated that TLR5 expression significantly decreases in the absence of Caveolin-1 expression in aging cells.[10] It is hypothesized that the Caveolin-1 directly interacts with TLR5 to stabilize it and hence increases the level of TLR5.
References
- Rock FL, et al. (1998). Proc Natl Acad Sci USA. 95 (2): 588–93.
- Stanislawowski M, et al. (2009). Journal of Physiology and Pharmacology. 60 Suppl 4: 71–5.
- Kim S, et al. (2014) J Immunol. 193(8):3902–13.
- Kassem A, et al. (2015). FASEB Journal. 29 (11): 4449–60.
- Husseinzadeh N, Davenport SM (2014). Gynecologic Oncology. 135 (2): 359–63.
- Smith KD, et al. (2003). Nature Immunology. 4 (12): 1247–53.
- Andersen-Nissen E, et al. (2005). Proc Natl Acad Sci USA. 102 (26): 9247–52.
- Gewirtz AT, et al. (2001). Journal of Immunology. 167 (4): 1882–5.
- Gohda J, et al. (2004). Journal of Immunology. 173 (5): 2913–7.
- Lim JS, et al. (2015). Molecules and Cells. 38 (12): 1111–7.
Be the first to review “Nori Salmon TLR5 ELISA Kit”
You must be logged in to post a review.























Reviews
There are no reviews yet.