Nori Human Pro-C3 ELISA Kit
$508.00 – $916.00
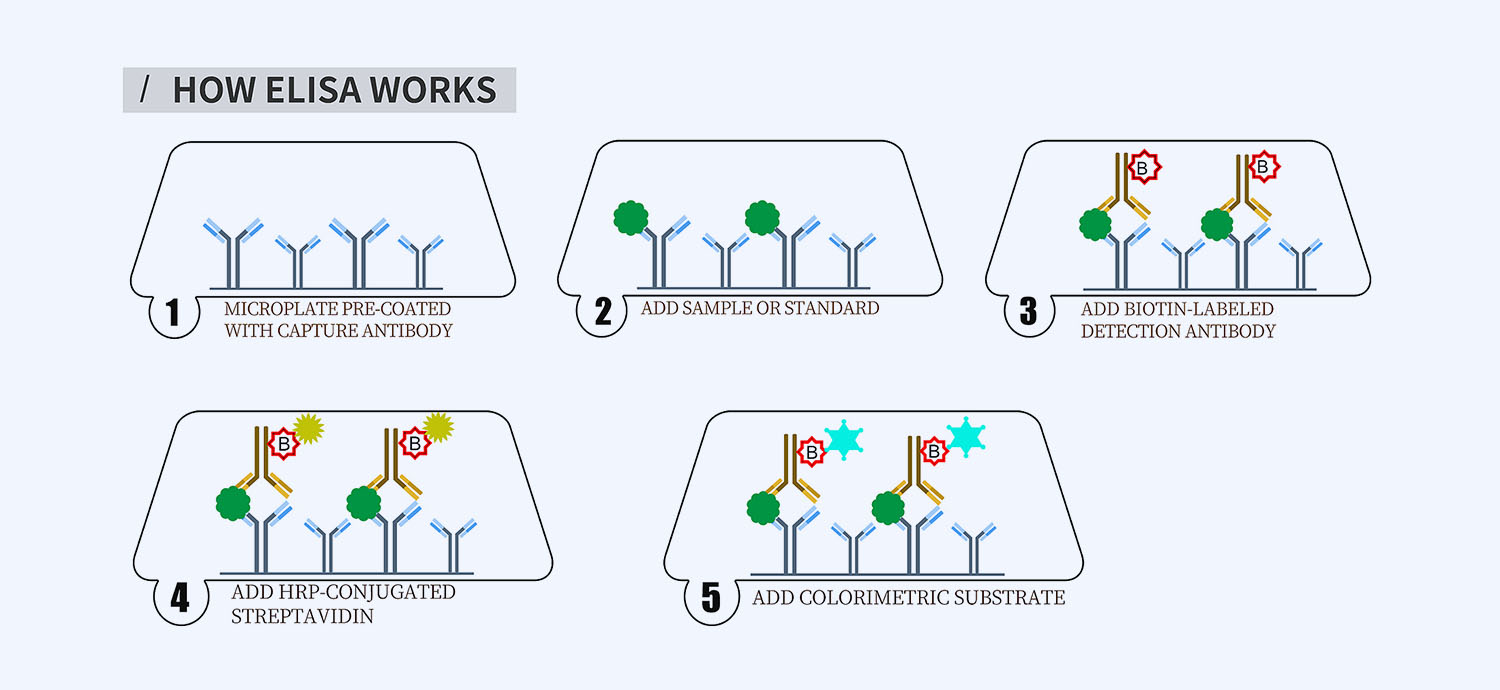
This ELISA kit is for quantification of Pro-C3 in human. This is a quick ELISA assay that reduces time to 50% compared to the conventional method, and the entire assay only takes 3 hours. This assay employs the quantitative sandwich enzyme immunoassay technique and uses biotin-streptavidin chemistry to improve the performance of the assays. An antibody specific for Pro-C3 has been pre-coated onto a microplate. Standards and samples are pipetted into the wells and any Pro-C3 present is bound by the immobilized antibody. After washing away any unbound substances, a detection antibody specific for Pro-C3 is added to the wells. Following wash to remove any unbound antibody reagent, a detection reagent is added. After intensive wash a substrate solution is added to the wells and color develops in proportion to the amount of Pro-C3 bound in the initial step. The color development is stopped, and the intensity of the color is measured.
Alternative names for Pro-C3: N-terminal pro-collagen III peptide
This product is for Laboratory Research Use Only not for diagnostic and therapeutic purposes or any other purposes.
- Description
- How Elisa Works
- Product Citation (0)
- Reviews (0)
Description
Nori Human Pro-C3 ELISA Kit Summary
Alternative names for Pro-C3: N-terminal pro-collagen III peptide
| Assay Type | Solid Phase Sandwich ELISA |
| Format | 96-well Microplate or 96-Well Strip Microplate |
| Method of Detection | Colorimetric |
| Number of Targets Detected | 1 |
| Target Antigen Accession Number | na |
| Assay Length | 3 hours |
| Quantitative/Semiquantitative | Quantitative |
| Sample Type | Plasma, Serum, Cell Culture, Urine, Cell/Tissue Lysates, Synovial Fluid, BAL, |
| Recommended Sample Dilution (Plasma/Serum) | No dilution for sample <ULOQ; sufficient dilution for samples >ULOQ |
| Sensitivity | 150 pg/mL |
| Detection Range | 0.78-50 ng/mL |
| Specificity | Human Pro-C3 |
| Cross-Reactivity | < 0.5% cross-reactivity observed with available related molecules, < 50% cross-species reactivity observed with species tested. |
| Interference | No significant interference observed with available related molecules |
| Storage/Stability | 4 ºC for up to 6 months |
| Usage | For Laboratory Research Use Only. Not for diagnostic or therapeutic use. |
| Additional Notes | The kit allows for use in multiple experiments. |
Standard Curve
Kit Components
1. Pre-coated 96-well Microplate
2. Biotinylated Detection Antibody
3. Streptavidin-HRP Conjugate
4. Lyophilized Standards
5. TMB One-Step Substrate
6. Stop Solution
7. 20 x PBS
8. Assay Buffer
Other Materials Required but not Provided:
1. Microplate Reader capable of measuring absorption at 450 nm
2. Log-log graph paper or computer and software for ELISA data analysis
3. Precision pipettes (1-1000 µl)
4. Multi-channel pipettes (300 µl)
5. Distilled or deionized water
Protocol Outline
1. Prepare all reagents, samples and standards as instructed in the datasheet.
2. Add 100 µl of Standard or samples to each well and incubate 1 h at RT.
3. Add 100 µl of Working Detection Antibody to each well and incubate 1 h at RT.
4. Add 100 µl of Working Streptavidin-HRP to each well and incubate 20 min at RT.
5. Add 100 µl of Substrate to each well and incubate 5-30 min at RT.
6. Add 50 µl of Stop Solution to each well and read at 450 nm immediately.
Background:
Pro-C3 (N-terminal pro-collagen III peptide) is a marker of fibrogenesis and formation of type III collagen neo-epitopes. During liver fibrosis, extracellular matrix proteins are degraded by matrix metalloproteinases into soluble fragments. PRO-C3 correlates to fibrosis stage and degree of portal hypertension in HIV/HCV co-infected patients [1]. A study showed that PRO-C3 correlates to histologic fibrosis progression in patients with chronic hepatitis C.[2]. PRO-C3 levels are associated with grade of fibrosis in Fibroscan in HIV mono-infected individuals.[3] Liver fibrosis and liver injury are reflected by PRO-C3 levels in HIV infected patients and can rule-out hepatic fibrosis in these patients. Moreover, PRO-C3 may be used to monitor patients at risk of fibrosis in centers where Fibroscan is not available. Pro-C3 is also associated with inflammation.[2] Pro-C3 differentiated mild/moderate from severe disease and, importantly, can identify CHC patients with fibrosis progression more accurately than the broadly used FibroTest.[4] Pro-C3 represents a rational blood parameter to be included in future studies in combination with other non-invasive modalities for monitoring disease progression for example in NASH, and eventually for assessing efficacy of potential antifibrotic therapies. PRO-C3 may be more reflective of the synthesis of type III collagen and fibrogenesis.[5] Patients with chronic hepatitis C who had higher baseline PRO-C3 levels (≥20.2 ng/ml) showed progression (worsening) of fibrosis while those with lower PRO-C3 did not progress.[5, 2] Further, PRO-C3, PRO-C5 and C4M have also been correlated with hepatic venous pressure gradient which is an invasive diagnostic and prognostic marker in cirrhosis for portal hypertension.[6,7] Higher PRO-C3 levels were associated with higher grade of lobular inflammation and ballooning, suggesting that PRO-C3 levels may be reflective of active disease. Furthermore, decrease of PRO-C3 over time was associated with improvement (or regression) in fibrosis.[8] unlike elastography which measures liver stiffness, PRO-C3 measures active collagen formation reflective of fibrogenesis, but not exactly the fibrosis area in the liver. PRO-C3 may be more valuable for identifying patients with active fibrogenesis than diagnosing static disease stages. Some patients with advanced fibrosis may have less active disease with old scars, and thus may have low levels of PRO-C3. This may explain the normal levels of PRO-C3 in some F3-4 patients.
References
- Jansen C, et al. (2014) PLoS One 2014, 9:e108544. https://doi.org/10.1371/journal.pone.0108544.
- Nielsen MJ, et al. (2015) Liver Int, 35:429–437.
- Dold L et al. (2019) PLOS ONE https://doi.org/10.1371/journal.pone.0219526
- Boyle M et al. (2019) JHEP Reports 1:188-198.
- Karsdal, M. A. et al. (2016) Am J Physiol Gastrointest Liver Physiol 311, G1009–G1017.
- Leeming, D. J. et al. (2013). Aliment Pharmacol Ther 38, 1086–1096.
- Leeming, D. J. et al. (2015) Scand J Gastroenterol 50, 584–592.
- Luo Y et al. (2018) Nature 8:12414. DOI:10.1038/s41598-018-30457-y.
Be the first to review “Nori Human Pro-C3 ELISA Kit”
You must be logged in to post a review.
























Reviews
There are no reviews yet.