Nori Equine Ghrelin ELISA Kit
$461.00 – $832.00
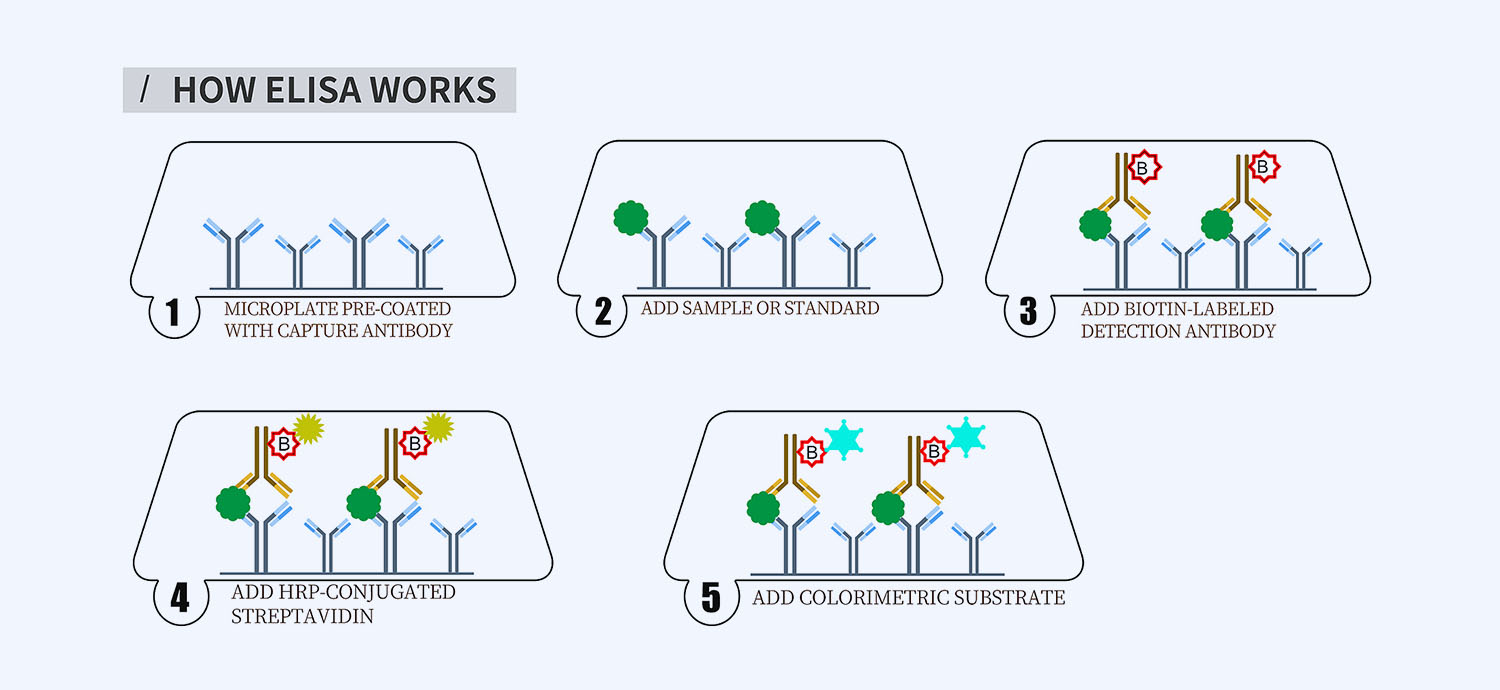
This ELISA kit is for quantification of GHRL in horse. This is a quick ELISA assay that reduces time to 50% compared to the conventional method, and the entire assay only takes 3 hours. This assay employs the quantitative sandwich enzyme immunoassay technique and uses biotin-streptavidin chemistry to improve the performance of the assays. An antibody specific for GHRL has been pre-coated onto a microplate. Standards and samples are pipetted into the wells and any GHRL present is bound by the immobilized antibody. After washing away any unbound substances, a detection antibody specific for GHRL is added to the wells. Following wash to remove any unbound antibody reagent, a detection reagent is added. After intensive wash a substrate solution is added to the wells and color develops in proportion to the amount of GHRL bound in the initial step. The color development is stopped, and the intensity of the color is measured.
Alternative names for ghrelin: GHRL
This product is for laboratory research use only not for diagnostic and therapeutic purposes or any other purposes.
- Description
- How Elisa Works
- Product Citation ()
- Reviews (0)
Description
Nori Equine Ghrelin ELISA Kit Summary
Alternative names for ghrelin: GHRL
Alternative name for equine: Horse
| Assay Type | Solid Phase Sandwich ELISA |
| Format | 96-well Microplate or 96-Well Strip Microplate |
| Method of Detection | Colorimetric |
| Number of Targets Detected | 1 |
| Target Antigen Accession Number | F6QE38 |
| Assay Length | 3 hours |
| Quantitative/Semiquantitative | Quantitative |
| Sample Type | Plasma, Serum, Cell Culture, Urine, Cell/Tissue Lysates, Synovial Fluid, BAL, |
| Recommended Sample Dilution (Plasma/Serum) | No dilution for sample <ULOQ; sufficient dilution for samples >ULOQ |
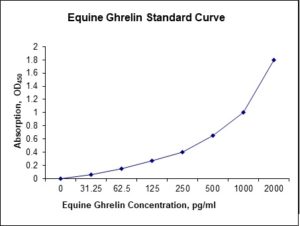
| Sensitivity | 6 pg/mL |
| Detection Range | 31.25-2000 pg/mL |
| Specificity | Equine ghrelin |
| Cross-Reactivity | < 0.5% cross-reactivity observed with available related molecules, < 50% cross-species reactivity observed with species tested. |
| Interference | No significant interference observed with available related molecules |
| Storage/Stability | 4 ºC for up to 6 months |
| Usage | For Laboratory Research Use Only. Not for diagnostic or therapeutic use. |
| Additional Notes | The kit allows for use in multiple experiments. |
Standard Curve
Kit Components
1. Pre-coated 96-well Microplate
2. Biotinylated Detection Antibody
3. Streptavidin-HRP Conjugate
4. Lyophilized Standards
5. TMB One-Step Substrate
6. Stop Solution
7. 20 x PBS
8. Assay Buffer
Other Materials Required but not Provided:
1. Microplate Reader capable of measuring absorption at 450 nm
2. Log-log graph paper or computer and software for ELISA data analysis
3. Precision pipettes (1-1000 µl)
4. Multi-channel pipettes (300 µl)
5. Distilled or deionized water
Protocol Outline
1. Prepare all reagents, samples and standards as instructed in the datasheet.
2. Add 100 µl of Standard or samples to each well and incubate 1 h at RT.
3. Add 100 µl of Working Detection Antibody to each well and incubate 1 h at RT.
4. Add 100 µl of Working Streptavidin-HRP to each well and incubate 20 min at RT.
5. Add 100 µl of Substrate to each well and incubate 5-30 min at RT.
6. Add 50 µl of Stop Solution to each well and read at 450 nm immediately.
Background:
Ghrelin, a hunger hormone, also known as lenomorelin, is a peptide hormone produced by ghrelinergic cells in the gastrointestinal tract.[1] Ghrelin functions as a neuropeptide in the central nervous system[2] and regulates appetite and energy homeostasis.[3] Ghrelin is encoded by the GHRL gene and is presumably produced from the cleavage of the prepropeptide ghrelin/obestatin. Full-length preproghrelin is homologous to promotilin and both are members of the motilin family. When the stomach is empty, ghrelin is secreted. When the stomach is stretched, secretion stops. It acts on hypothalamic brain cells both to increase hunger, and to increase gastric acid secretion and gastrointestinal motility to prepare the body for food intake.[4] Ghrelin was found to be upregulated by stress even in the absence of adrenal hormones.[5] The receptor for ghrelin, the ghrelin/growth hormone secretagogue receptor, is found on the same cells in the brain as the receptor for leptin, the satiety hormone that has opposite effects from ghrelin.[5] Ghrelin also regulates reward cognition in dopamine neurons that link the ventral tegmental area to the nucleus accumbens[6] through its colocalized receptors and interaction with dopamine and acetylcholine.[2] Unlike the case of many other endogenous peptides, ghrelin can cross the blood-brain-barrier, giving exogenously-administered ghrelin unique clinical potential. In addition to its function in energy homeostasis, ghrelin also activates the cholinergic–dopaminergic reward link in inputs to the ventral tegmental area and in the mesolimbic pathway,[6] a circuit that communicates the hedonic and reinforcing aspects of natural rewards,[2] such as food and addictive drugs such as ethanol. Ghrelin receptors are located on neurons in this circuit.[2] Hypothalamic ghrelin signalling is required for reward from alcohol and palatable/rewarding foods. Ghrelin has been linked to inducing appetite and feeding behaviors. Circulating ghrelin levels are the highest right before a meal and the lowest right after.[7] Injections of ghrelin in both humans and rats have been shown to increase food intake in a dose-dependent manner.[8] However, ghrelin does not increase meal size, only meal number. Studies have shown that ghrelin levels are negatively correlated with weight. This data suggests that ghrelin functions as an adiposity signal, a messenger between the body’s energy stores and the brain.[4]
References
- Inui A, et al. (2004). The FASEB Journal. 18 (3): 439–56.
- Dickson SL, et al. (2011). Molecular and Cellular Endocrinology. 340 (1): 80–87.
- Burger KS, Berner LA (2014). Physiology & Behavior. 136: 121–27.
- Schwartz MW, et al. (2000). Nature. 404 (6778): 661–71.
- Perello M, et al. (2012). The Journal of Comparative Neurology. 520 (2): 281–94.
- Naleid AM, et al. (2005). Peptides. 26 (11): 2274–79.
- Tolle V, et al. (2002). Endocrinology. 143 (4): 1353–61.
- Wren AM, et al. (2000). Endocrinology. 141 (11): 4325–28.
Be the first to review “Nori Equine Ghrelin ELISA Kit”
You must be logged in to post a review.






















Reviews
There are no reviews yet.